Every day, our bodies wage a silent, yet constant and relentless, even fierce, war. On the one hand, pathogenic viruses, bacteria, and other invaders. On the other, a complex immune system that strives to recognize, attack, and destroy the invader.

Source: Pixabay
But there's something else: a system that ensures this defensive army doesn't turn against its own "country"—that is, against our own bodies. In 2025, the Nobel Prize in Medicine or Physiology was awarded to three scientists for the discovery of precisely this surveillance system—the peripheral (immune) tolerance system.
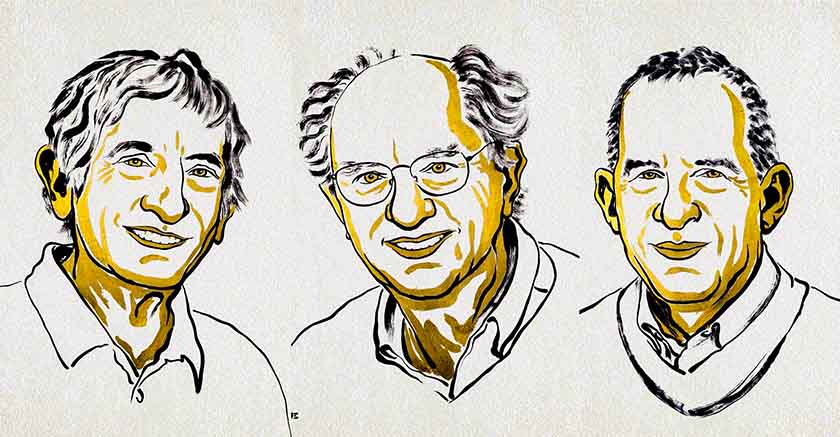
The laureates were Mary E. Brunkow (USA), Fred Ramsdell (USA), and Shimon Sakaguchi (Japan). They received the award "for their discoveries concerning peripheral immune tolerance."
What was the defense vision so far?
Until now, it was believed that the body's main tool for preventing itself from attacking itself was central tolerance—the elimination of immune cells that recognized its own tissues as the enemy, starting in the thymus gland. To put it simply: we teach soldiers (immune cells) in the training center (thymus) not to shoot at their own.
But this vision didn't explain everything. In practice, some people, despite this mechanism, developed autoimmune diseases—the body began attacking its own cells: joints, thyroid, pancreas, muscles, nerves. Therefore, something—beyond central tolerance—would have to act as a brake, a guard, when the thymus's training failed.
Discovering the Guardians of the Immune System
The first laureate, Shimon Sakaguchi, worked in the mid-1990s and demonstrated that there is a group of mature T lymphocytes that acts as a regulator, inhibiting the excessive activity of other immune cells. These cells were called regulatory T cells (T-regs). This gave rise to the idea of peripheral tolerance—that is, not only the elimination of inappropriate cells in the thymus protects against autoimmunity, but also active inhibition in an "additional mechanism"—at the periphery of the immune system.
Then, in 2001, Mary Brunkow and Fred Ramsdell described a mutation in the FOXP3 gene that led to a severe autoimmune disease in mice ("scurfy mouse"). Consequently, they determined that in humans, mutations in this gene lead to IPEX (Immune dysregulation, Polyendocrinopathy, Enteropathy, X-linked) syndrome—a rare, fatal immune system disorder. Sakaguchi later demonstrated that FOXP3 controls the development of T-regulators—completing a crucial cycle.
As a result, we now understand that our body has not one, but at least two control systems – central and peripheral – which ensure the relative safety of its own tissues.
Why does this matter?
On a scientific level, the discovery is significant because it demonstrates that immunology must be viewed as a complex, dynamic network with brakes and accelerators, not just as a simple "attack and defense." On a practical level, the implications are enormous.
First, we have a better understanding of autoimmune diseases—such as type 1 diabetes, multiple sclerosis, rheumatoid arthritis, and lupus—where the immune system attacks its own body. This discovery provides tools for better understanding the causes and potential treatments.

Announcement of the Nobel Prize in Physiology or Medicine for 2025. Photo: Cecilia Odlind (Source: Karolinska Institutet)
Secondly, the discovery has implications for transplantology – if T-regs could be activated or increased, it might be possible to suppress graft rejection. On the other hand, in oncology – where the immune system should attack cancer cells – the opposite may be true: inhibiting T-regs could help the body fight cancer.
Thirdly, the discovery opens the door to numerous clinical trials – there are already over 200 ongoing clinical trials of T regulatory cell therapies.
What's next – a step towards the medicine of the future?
Although this triumph of science has been officially recognized, the road to full clinical application is still long. As is often the case in medicine, the idea is great, but implementation requires time, money, and careful consideration.
Questions arise regarding T-regulators: how can we safely increase their number or activity in patients with autoimmune diseases? How can we avoid excessive immune tolerance allowing cancer or infection to develop? How can we tailor therapy to a specific patient, given that everyone's immune system is different? These are the challenges facing immunotherapy.
In oncology, on the other hand, the opposite is true: inhibiting T-regulators can increase the immune system's ability to attack cancer cells. But here, too, many mysteries remain: what will the side effects be? Will there be an excessive immune response, damaging the body's own tissues?
It is also worth noting that the scientific method that led to this discovery may inspire other fields of medicine – immunology is increasingly becoming the “centre” of therapeutic activities.
What does it look like from a practical perspective for the patient?
For patients, this means hope—not immediate, but real. In autoimmune diseases, immunosuppressive drugs (those that limit the immune response) are currently used, but these often have side effects, including susceptibility to infection, risk of cancer, and general weakness. Therapies based on activating T-regs could be more targeted, "strengthening the system's guards" rather than suppressing the entire army. For transplant recipients, it also signals that the time may come for more precise therapies that will allow the transplanted organ to survive longer with lower drug doses.
For a healthy person, it's a reminder that our body has a complex regulatory system; that balance is crucial – too weak an immune response = infections, too strong = autoimmunity. The laureates' discovery suggests that the "guardians" of this system are real – and they can be modulated in the future.
Winners – short profiles

Brunkow, Ramsdel, Sakaguchi - The Nobel Prize in Medicine 2025 (Source: Nobel Committee/Niklas Elmehed)
Mary E. Brunkow was born in 1961, received her doctorate from Princeton, among others, and conducted her research in Seattle, at the Systems Biology Institute.
Fred Ramsdell born in 1960, received his doctorate at the University of California, Los Angeles (UCLA), and then worked, among others, at Sonoma Biotherapeutics in San Francisco.
Shimon Sakaguchi, born in 1951, professor at Osaka University (Japan), started research in the 1990s.
They were jointly awarded on October 6, 2025. The prize is 11 million Swedish kronor (USD 1.17 million) and will be divided among them.
Conclusions for Poland and the world
Although this year's Nobel Prize was awarded to scientists from the US and Japan, Polish immunology has been developing for years in sync with global science and has achieved notable results of its own, which, from today's perspective, take on new significance. As early as the 1970s and 1980s, researchers in Warsaw, Wrocław, and Kraków conducted pioneering work on T lymphocytes, delayed-type hypersensitivity reactions, and autoimmunity, utilizing small but creatively utilized laboratories. Today, the heirs of those traditions are young research teams beginning to specialize in precise methods of analyzing the immune system: single-cell RNA-seq, high-dimensional cytometry, superresolution microscopy, and immunological bioinformatics.
Polish projects connecting research laboratories with clinics also play a significant role. Medical universities in Warsaw, Gdańsk, and Poznań are developing research on immune regulation in skin and thyroid diseases, as well as hematological malignancies. Oncology centers in Gliwice and Warsaw are analyzing the role of regulatory T cells in the tumor microenvironment, searching for signals that could increase the effectiveness of immunotherapy. The Transplantology Clinic in Warsaw is also conducting projects on the early detection of immune tolerance disorders in transplant recipients.
While this may sound modest compared to global giants, Polish immunology is attempting to build its own technological and human resources. Young researchers are increasingly returning from abroad with new competencies, and international grants and consortia offer the opportunity for Polish laboratories to become part of a larger puzzle. The discovery of T-regs demonstrates that this field doesn't require huge budgets—it requires patience, ingenuity, and long-term support.
In Poland, research on immune regulation is developing, among other things, in the context of type 1 diabetes, one of the most common autoimmune diseases in children and adolescents. Research teams in Warsaw and Łódź are analyzing the function of regulatory lymphocytes in patients at an early stage of the disease, searching for biomarkers that will predict its progression. Thanks to the discoveries of this year's Nobel Prize winners, new Polish projects can be more quickly incorporated into international clinical trials.
Poland, as a country, can and should draw conclusions. First, investing in basic science pays off. Biochemical and immunological research at the molecular level leads to discoveries that are important for all of humanity. Second, international cooperation, access to data, and the exchange of experiences are crucial in the era of global medicine. Third, public education – it's worth explaining that the immune system is not just about fighting and threats, but also a delicate balance, the mechanisms of which are still being discovered.
For Poland, this may also be a signal for the healthcare and research system: it is worth supporting immunology, T-cell research, creating conditions for start-ups and greater links between science and the medical industry.
From a Polish perspective, it's particularly valuable that discoveries regarding peripheral tolerance are opening up new areas for research already underway in the country. This applies to both autoimmune diseases—very common in the Polish population—and oncology, where immunomodulatory therapies are becoming a cornerstone of treatment.
Polish scientists are beginning to specialize in analyzing the immunological profiles of specific patients, which in the future will allow for greater precision in selecting therapies. It is here—at the intersection of clinic and laboratory—that a space may emerge where Polish science can play a significant role.
Summary
The year 2025 marked the history of medicine with a discovery that transformed our understanding of the immune system: it is not simply a collection of cells attacking intruders, but also a system with its own guardians—regulatory T cells—that prevent attacks on its own tissues. Mary E. Brunkow, Fred Ramsdell, and Shimon Sakaguchi, recognized for this very reason, demonstrated that the mechanism of peripheral tolerance is crucial for health and that its disruption can be the root of autoimmune diseases. This discovery opens the door to a new generation of therapies—in autoimmunity, transplantology, and oncology—though the path from the laboratory to the clinic can be long. For all of us, it is a reminder of how complex, beautiful, and fragile our own defense system is.