Today in "Kuryer Polski," we take a closer look at this year's Nobel Prize in Chemistry—a prize that has the potential to transform the landscape of chemistry, materials technology, and the environment. It's not only a great honor for the laureates, but also a signal that chemistry is entering new, exciting stages of development. We explain how this happened, what the award-winning work entails, and why it may also be significant for the average person, not just for laboratories.
Illustration by A. Wozniewicz
The Award Goes to... Holes?
In the world of science, it's rare for an invention to be simultaneously beautiful, practical, and filled with poetic symbolism. This year's Prize is one such exception. It's a story in which, as one member of the Nobel Committee put it, "the holes became an asset".
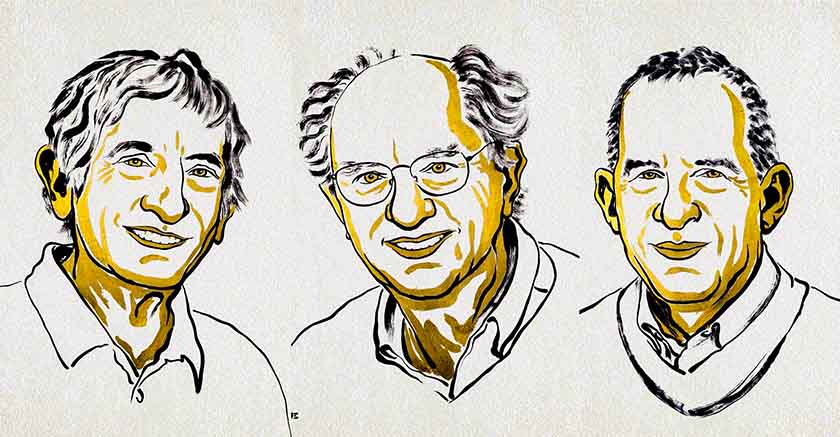
The 2025 Nobel Prize in Chemistry was awarded to three scientists:
Susumu Kitagawa (Kyoto University, Japan)
Richard Robson (University of Melbourne, Australia)
Omar M. Yaghi (University of California, Berkeley, USA)
The laureates were recognized "for the development of metal–organic frameworks," known as MOFs (metal–organic frameworks). Their work is a quiet but profound revolution, transforming the way scientists understand and construct matter. In short, they have developed a new molecular architecture—a combination of metal ions and organic molecules, forming crystalline networks with very large cavities (pores) within them, with metal ions as nodes and organic molecules as connectors. It's a kind of crystalline sponge, or foam.
What is the essence of work?
To explain it simply: imagine a small container that appears small from the outside, but inside has lots of microscopic tunnels and chambers – so it can store a significant amount of gas or liquid.
In MOF structures, metal ions act as the "nodes" of the structure, and organic molecules ("arms", or edges) connect these nodes into a network.
Effect: a material with a very large internal surface area – in terms of mass, it can store a lot of gas or liquid molecules.
Example: one gram of such a material — the size of a typical sugar cube — would have a pore area equal to the size of a football field.
Applications already discussed by the Nobel Academy include: CO₂ capture, water extraction from very dry air, toxic gas storage or separation.
The media has colloquially adopted the metaphor: "small structure, huge capacity" - they even compare the MOF material to Hermione Granger's handbag from "Harry Potter", which, although it looks ordinary, holds an extraordinary amount of things.
From Chaos to Order – How MOFs Were Born
By the end of the 20th century, materials chemistry had already achieved a wealth of achievements. Crystals, polymers, catalysts, and semiconductors could be created. But something was missing: a universal language of molecular construction, a kind of chemical LEGO, where ions and molecules could be used to build materials with precisely defined properties.
This is precisely what the laureates proposed. Their idea—to connect metal ions (nodes) with organically constructed linkers (arms)—resulted in regular crystal lattices with a gigantic internal surface area. At the atomic level, such structures create microscopic tunnels and pockets—pores that can store, filter, and even separate gas or liquid molecules.
"A gram of MOF, ... looks like a sugar cube - it has an internal surface area the size of a football field"
– explained Omar Yaghi with a smile in an interview with the Wall Street Journal.
In the 1990s, no one imagined that these "lattice" structures would inspire thousands of laboratories around the world. Today, we know of over 100,000 different MOFs, each with a different geometry and function.
It is worth quoting Yaghi himself:
“You take molecular building blocks and connect them together to create scaffolds with enormous surface area and highly tunable chemical properties.”
And that word "tunable" is key. It's not just about the material that will work—it's about the material whose properties can be fine-tuned: which metal, which connector, what pore size—all of these things allow for the ability to "tailor the material for a specific task."
Three winners – three continents, one idea

Susumu Kitagawa, Richard Robson, and Omar M. Yaghi (Illustration: Niklas Elmehed, Nobel Prize Committee)
Let's take a look at this year's three winners:
Susumu Kitagawa
A Japanese chemist from Kyoto University was one of the first to recognize the potential of "porous" chemistry. He coined the term "soft porous crystals"—soft, dynamic materials that can respond to changes in pressure or temperature.
Kitagawa said:
My dream is to capture air, separate oxygen, nitrogen, carbon dioxide from it and turn it all into something useful.
Richard Robson
An Australian from the University of Melbourne, he built the first models of metal-organic networks in the 1980s. His work was pioneering, though it remained in the shadows for a long time. Today, it forms the theoretical foundation of the entire field.
Omar M. Yaghi
A professor at Berkeley of Jordanian descent, he was charismatic, brilliant, and also deeply humanistic. He introduced the concept of "reticular chemistry"—a framework chemistry where molecules, like a LEGO cube, are constructed—Yaghi himself used this metaphor. It's an approach in which matter is treated like architecture: atoms are used to construct structures with desired functions.
Yaghi also gained notoriety for his experiments on extracting water from desert air. His prototype devices, based on MOFs, can condense moisture even at 15% humidity. This could be a breakthrough technology for regions like North Africa and the Middle East.
What can MOFs actually do?
Carbon Dioxide Capture MOF structures can selectively capture CO₂ molecules from gas mixtures, such as air. They can be used in power plant chimneys, industrial installations, and even personal devices.
Water Recovery from Air Special MOFs, such as MOF-801, absorb water vapor at night and release it during the day under the influence of the sun's heat. This can help recover water in arid regions—an increasingly important task in times of drought and water shortages. This is no longer theoretical—such devices are operating in Berkeley and Dubai.
Energy Storage Thanks to their enormous internal surface area, MOFs can store hydrogen and methane in a safe, compact form. This offers an opportunity for the development of hydrogen transport.
Purification and Filtration Some of them bind toxins, heavy metals, and chemical warfare gases. The chemical and military industries are already testing these solutions.
Medicine and Biotechnology MOFs can be loaded with drug molecules and their release rate can be controlled. They're like "smart capsules" that know when to open.
New Material Technologies Thanks to the ability to design these structures—selecting metals and organic "connectors"—it becomes possible to create materials tailored to a specific function. This means that for a given task, you can design MOFs with optimized properties.
Breakthrough in Chemistry The award demonstrates how chemistry can go beyond pure "research," beyond typical test-tube reactions. It's not just about understanding reactions in a flask, but about creating entirely new structures with real application potential—this is molecular architecture.
Chemistry with soul – a combination of science and art
While it sounds technical, there's something deeply humanistic about the laureates' work. Yaghi often says:
Chemistry is beauty. When you look at a crystal and see perfect order emerge from chaos – that is poetry.
It's extraordinary: a scientist who speaks of the poetry of structures while simultaneously creating solutions to global problems. It was this balance between aesthetics and practice that captivated the Nobel Committee.
Understanding the World Through Its... Holes
Scientists love numbers. And the numbers in this case are impressive:
Specific surface area of MOF-5 ≈ 2900 m²/g; MOF-5 is one of the recognizable MOF structures (Zn₄O(BDC)₃).
It exhibits permanent porosity and the pore density is approx. 50% by volume,
Adsorbed gas volume – up to 800 cm³/g.
But behind these numbers lies something deeper: the idea of controlling space at the atomic level. If we can design a void that only accepts specific particles, then we enter the era of programmable matter.
“This year’s prize is a story full of holes – but with enormous potential to absorb your full attention.” – Olof Ramström, Nobel Committee
Curiosities and anecdotes
“Hermione's Handbag” – this is how The Guardian titled its article, comparing MOFs to the magical bag from Harry Potter: inconspicuous from the outside, bottomless inside.
Omar Yaghi had been nominated for a Nobel Prize before – he was referred to as the "chemical architect of the 21st century".
Susumu Kitagawa started as a catalysis researcher and ended up in MOFs… by accident. "It was supposed to be a supporting position for a PhD student," he joked after the award announcement.
For years, Richard Robson didn't have a large team. He worked with a few students and makeshift equipment. It wasn't until twenty years later that his work began to be cited hundreds of times a year.
Why is this a Nobel-worthy breakthrough?
They changed the way we think about chemistry. Previously, chemists were more of observers—studying how substances react. Now they become architects who design reactions and space.
They combined science with engineering. MOFs bridge theory and practice—from quantum models to industrial filters. Chemistry is combined with physics, materials science, and environmental technologies.
They opened a new field. "Reticular chemistry", the chemistry of lattices, is today a separate field of research.
They showed the global dimension of science. Japan, Australia, USA – three different continents, three different schools, one language of molecules.
Polish perspective
What does this mean for us in Poland? Although the award is presented to scientists from Japan, Australia, and the USA, its implications are global – including for Poland:
Applications in industry and the environment: Technologies based on MOFs can also be implemented in Poland – e.g. in air purification, gas storage, water recovery.
A challenge for Polish chemistry and materials science: This is a signal that Poland can and should invest in research on advanced materials to become not only a user, but also a creator.
Education and the Future: This is an opportunity for young people and young scientists to see that chemistry isn't just about pipettes and flasks—that it's about building the future. MOFs are an open field—they can combine chemistry, physics, engineering, and computer science.
Science-Industry Collaboration: The coming era of functional materials requires collaboration between universities, research institutes, and industry. Poland has the potential to excel in this area.
Although this year's award doesn't have a direct "Polish" theme, its significance for our country is clear. Poland has a strong chemical tradition, from Marie Skłodowska-Curie to contemporary centers in Kraków, Warsaw, and Poznań. Research on MOFs for CO₂ capture is already underway at institutes of the Polish Academy of Sciences.
A Look into the Future
MOFs are just the beginning. Scientists are already creating their successors: COFs (covalent organic frameworks) and ZIFs (zeolitic imidazolate frameworks). All share the same idea: controlled matter design.
“In the future, materials will be as programmable as software.” – Omar Yaghi
It sounds like science fiction, but the first steps have already been taken. If the design and synthesis of MOFs can be automated, chemistry will become as digital as computer science.
Scale and Production
Of course, there are challenges: laboratory research is one thing, industrial scale is another. Many MOF materials work well, but the road to mass production, low cost, and industrial integration can be long. The media notes that "the issue now is simply engineering."
For Poland, this is a potential area where investments in research and production infrastructure and technology transfer can bring clear results.
Summary
The 2025 Nobel Prize in Chemistry is not only an honor for three outstanding scientists but also a signal that modern chemistry is addressing global challenges: climate, water, and resources. Their work—on the structures of metal-organic meshes—could in the future transform how we use materials and how we address environmental issues.
The 2025 Nobel Prize in Chemistry is a triumph of the idea that science doesn't have to be spectacular to be revolutionary. Three researchers, working for decades in different parts of the world, combined a simple idea with brilliant execution. From ions and molecules, they created something more than just materials—they created the language of a new chemistry. Such groundbreaking work often takes years to gain recognition, and this was the case here: Robson's first work dates back to the 1980s and 1990s.
Today, their work inspires environmental engineers, architects, biologists, and even economists who see the potential for a new, cleaner economy. For us, it's a reminder that science is an everyday reality, that tomorrow's solutions can be created today. It's worth following how this award-winning technology develops and asking how it can be used locally, in Poland.